04 Mar 2019 Early Biosimilars Pave the Way for Recent Launches in Europe
Introduction
With over a decade of real world biosimilar experience in Europe and now more than 50 biosimilars approved by the EMA, this edition of the NeuClone Biosimilar Brief looks at the long-term trends of Europe’s earliest biosimilar products and how these have paved the way for recent launches of more complex biosimilars throughout Europe.
Long-Term Trends: Total Volume Growth and Biosimilar Market Share
Three biologic molecules have faced biosimilar competition in Europe for over a decade. Somatropin was the first molecule exposed to biosimilar competition after Sandoz’s Omnitrope biosimilar received EMA approval in 2006. Epoetin biosimilars followed next in 2007 and third filgrastim biosimilars from 2008.
The well-known rationale behind biosimilar introduction is to provide high-quality, affordable alternatives to novel biologics that reduce healthcare expenditure and increase the number of patients able to access biologic treatments.
A report from IQVIA (2018) has tracked biosimilar competition in Europe, particularly changes in total volume and market share following biosimilar introduction. The report highlights that after biosimilars referencing filgrastim, epoetin and somatropin entered Europe, total volume (originator and biosimilars) increased across 23 European countries as seen in Exhibit 1. Most notable was a 134% increase in filgrastim total volume. Across the three molecules, greater biosimilar market share correlated to a larger expansion of the total market.
Exhibit 1: Total volume change and market share following biosimilar introduction.
Filgrastim, Epoetin and Somatropin markets across 23 European countries. Source: IQVIA (2018)
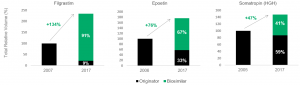
Key takeout 1: All three molecules achieved significant market expansion following biosimilar introduction.
The IQVIA report also reports biosimilar market dynamics on a country-specific basis, highlighting noteworthy outcomes in certain regions. For example, countries with low GDP/capita experienced some of the largest increases in total volume and biosimilar market share as the incentive to switch to the discounted biosimilar molecule is likely greater. In certain Eastern European countries such as Bulgaria, Hungary and Slovakia, filgrastim biosimilar introduction resulted in multiple-fold market expansion as well as large biosimilar market shares of up to 100% (see Exhibit 2). Such large market shifts were predominantly due to little or no recorded use of biologics prior to the introduction of biosimilars.
Exhibit 2: Total volume change and market share following biosimilar introduction.
Filgrastim in Bulgaria, Hungary and Slovakia. Source: IQVIA (2018)
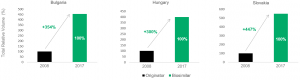
Key takeout 2: Underserved patient populations in low GDP/capita nations experienced the greatest increases in treatment due to biosimilar introduction.
Recent Launches: Uptake is Occurring at a Faster Rate
The experience gained from successful implementation of early biosimilar markets across Europe appears to have helped foster a conducive environment for the launch of recent biosimilars. More complex monoclonal antibody biosimilars of infliximab, rituximab, trastuzumab, and adalimumab, as well as fusion proteins such as etanercept, have demonstrated substantial uptake in relatively short time periods.
For example, South Korean biosimilar developer Celltrion, with the help of various commercialisation partners, has brought to market several of these later biosimilars in Europe. First launched was their infliximab biosimilar in 2013, reported to have captured 56% of the European infliximab market as of the 3Q2018. Next, Celltrion launched a rituximab biosimilar in 2017, capturing 35% of the rituximab market across 16 European countries by 3Q18 at a rate three to four times faster than their infliximab launch.
The much-anticipated expiry of Humira (adalimumab) and Herceptin (trastuzumab) patents resulted in biosimilars launching across Europe in 2018. In the short period of time these two biosimilar markets have been active, uptake appears to be occurring at a faster rate than any previous biosimilar. Trastuzumab biosimilars have gained 14% of the EU trastuzumab market in only seven months. Meanwhile, the four adalimumab biosimilar entrants currently in Europe have also reported strong early uptake across the EU5 with 15% of total adalimumab psoriasis use after just two months. So strong has the early adalimumab biosimilar uptake been that certain analysts believe its reasonable 50% of Humira use in Europe will be captured by biosimilars within the first year of competition.
Key takeout 3: Biosimilar uptake is occurring at a faster rate with each subsequent molecule exposed to competition.
Notably, with each subsequent launch, the rate of uptake appears to be occurring at a faster rate as confidence and experience with biosimilars grows. Previous concerns of product safety and quality have gradually been dispelled as more patients are treated in real world settings. Systematic reviews highlighting the safety of biosimilar switching, such as the Cohen et al. report tracking 14,225 unique individuals further improves stakeholder confidence to help stimulate biosimilar adoption.
It is important to recognise the heterogeneity of the European biosimilar market. No product has achieved consistent uptake across Europe and no one country as achieved consistent uptake across different molecules. Variation between countries and products is largely due to different payer-purchasing mechanisms, retail versus hospital usage, and the degree of competition among other factors.
Conclusion
The successful introduction in Europe of filgrastim, epoetin and somatropin biosimilars over a decade ago has expanded patient access to biologic treatments through high-quality, affordable alternatives. The resulting increase in stakeholder confidence has helped spur uptake of recent biosimilar introductions of more complex biologic molecules. Early adoption of these recent biosimilar products across Europe provides optimistic belief that in another decade, these recent entrants will result in even greater benefits to patients and lead what is estimated to be over an $11 billion European biosimilar market by 2024.
Contact: info@neuclone.com